2) IDMP+ enabled
Background
Past, this global industry segment started in 1993 with the introduction of electronic document management systems (EDMS) as point solutions. This was already great progress versus paper. In 1998 Industry and Regulators jointly formed the International Conference on Harmonization (ICH) to develop global communication standards (eCTD, electronic Common Technical Document) with an XML backbone, and pushed its implementation alongside with other relevant standards like medical terms (e.g. MedDRA, SNOMED, CDISC etc.).
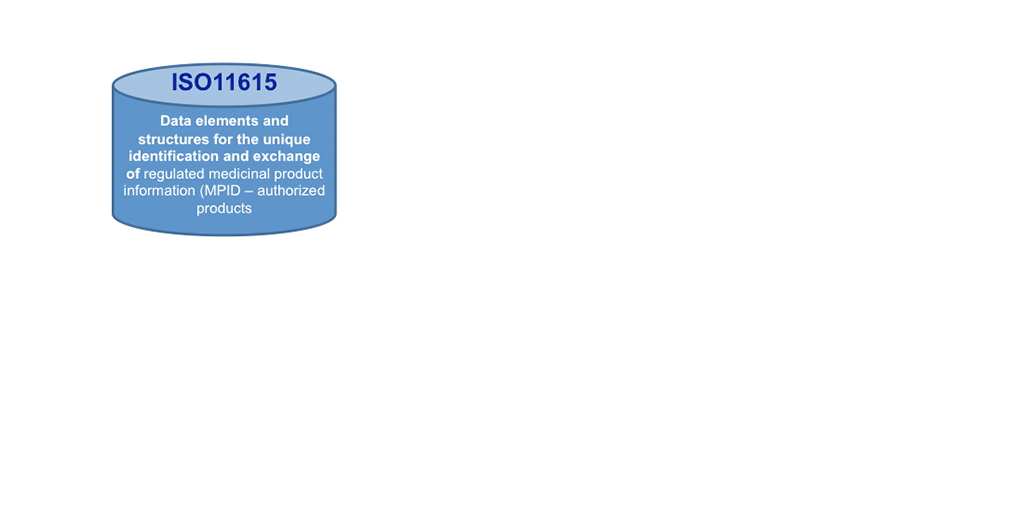
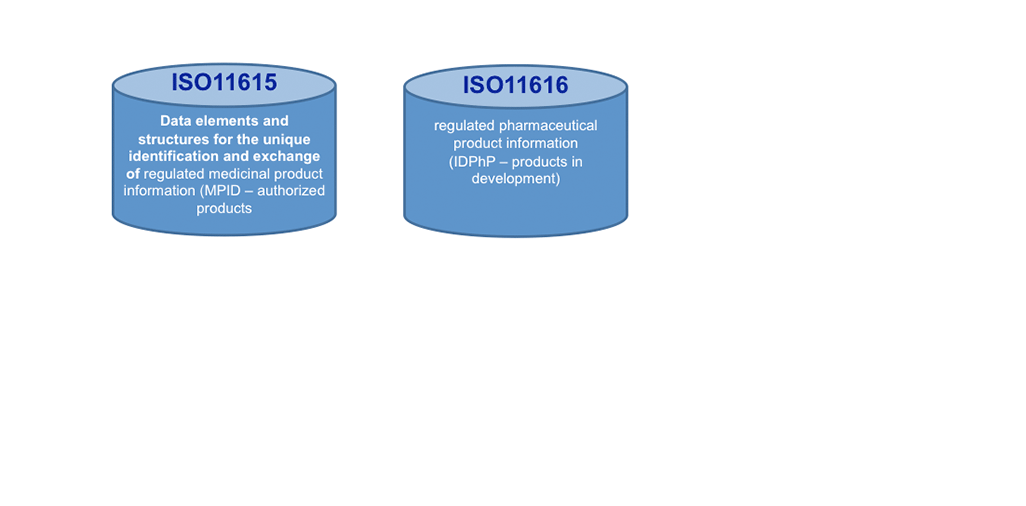
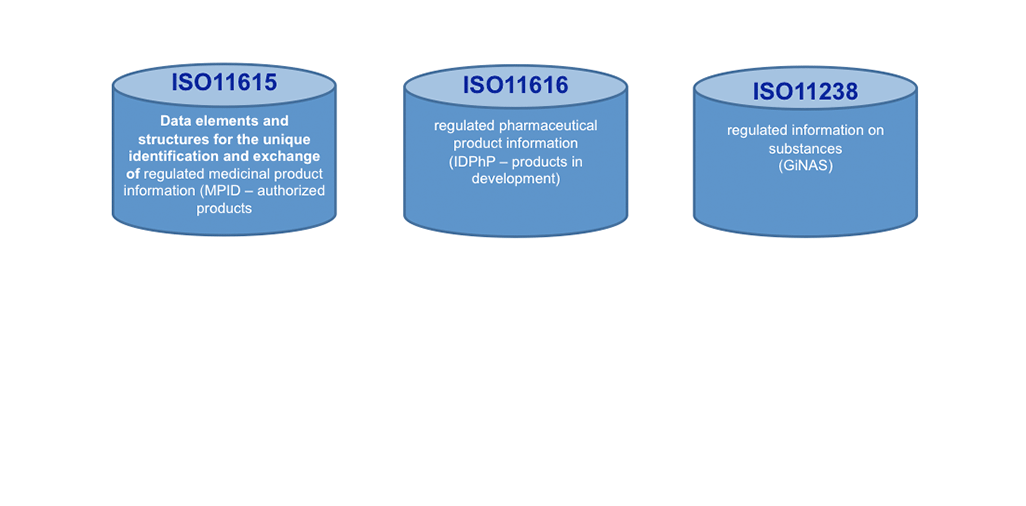
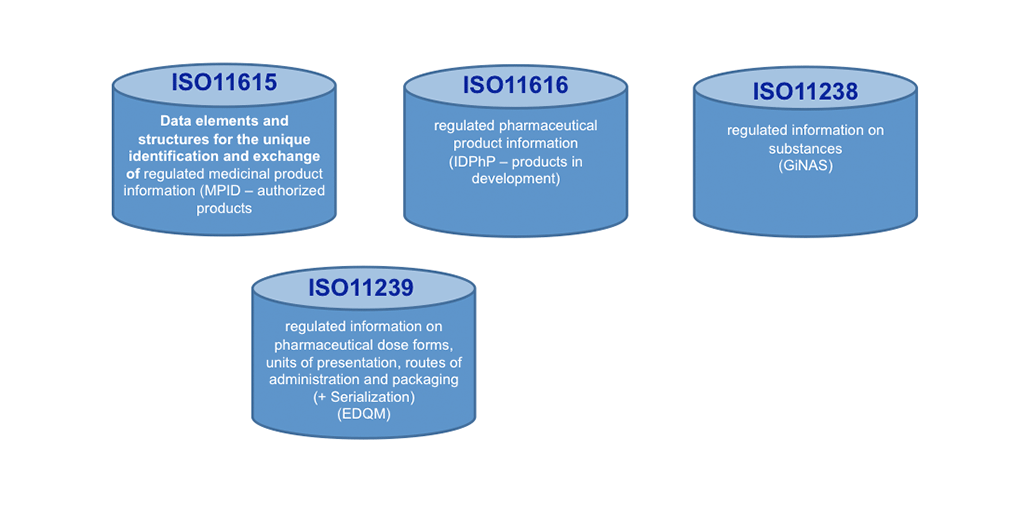
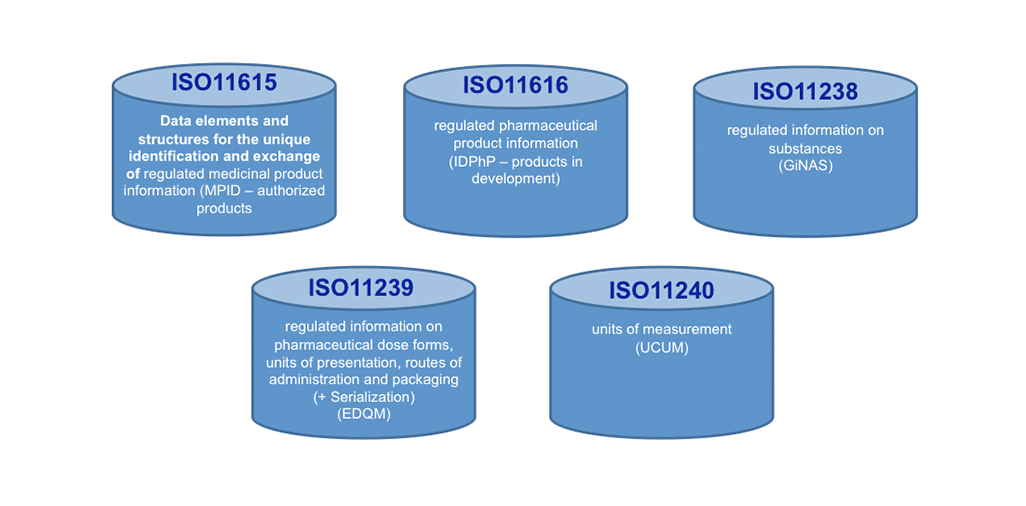
Today, ISO IDMP+ standards are on the horizon: These standards will help making a big move forward in KM, once implemented. We see here not only Controlled Vocabularies (CVs, ISO 11239 ) as one of the 5 IDMP+ Standards to support KM processes and making outcomes actionable, but the Unique Substance Code (ISO11238) offers to go the path of the Single Source in the lifecycle of Product Information. It will help search engines to become much more effective and faster!
View IDMP+ Standards as «Information Containers» that have Dependencies and are well structured: Adaption required!
Within those Containers there are given, stringent structures that will impact Information Architecture & Processes (level of granularity and re-use of components)
As of today, EMA plans enforcement of ISO IDMP+ Q4, 2018 for Iteration 1, with other Regions/countries to follow «instantly»
FACT: Introducing globally valid semantics will finally bring real KM forward, but it has no value, if not applied!
Latest Experience in the global Life Sciences Industry and their Regulators
Benefits from the global ISO Standards Virtual Knowledge Management (KM)
IDMP+ refers to ISO IDMP, ICSR & SPL Standards