1) Enterprise Data Governance (EDG)
An «Ideal Momentum»
The Pharmaceutical Industry and Health Authorities (HA) have undertaken an effort to harmonize global data by the implementation of the new ISO Identification of Medicinal Products (IDMP+) and related Standards. These additional standards include the ISO Individual Case Safety Reports (ICSR) and ISO Structured Product Labeling (SPL) and are herein collectively called IDMP+. All three are aimed at improving communication and patient safety on a global scale.
In order to satisfy the governance of data according to LSCP’s approach to IDMP+, higher content granularity is required, leading to an increased number of information components and associated metadata. The move to manage a large number of smaller documents requires a thorough revision to Enterprise Information Management, which includes Data Governance and Quality considerations across three major dimensions:
- Recognizing the value of information as an asset;
- Elevating standards not as a burden, but as a real business opportunity
- Strategizing the cross-functional application of IDMP+
This paper provides an overview of the IDMP+ approach that will address each of these areas as they should be covered in an Enterprise Information Management Process.
LSCP has teamed up with Prof. Dr. Peter Aiken to create this approach in the context of IDMP+. It is critical to get this project organized and executed in a pragmatic way with actionable data & information and clear, documented decisions!
- A globally recognized Data Management “guru”, strategically partnering with LSCP (since 2016) to add LS business, regulatory and process knowledge
- Our mission is to unlock the potential of organizational data assets
- We believe that data is the most powerful, yet underutilized and managed asset in organizations today
- Mapping/analyzing: e.g. identify data structures within IDMP data elements to enable optimal data re-use by applying Structured Component Management (SCM)
- However, a pre-requisite is a sufficient data literacy for which courses are available
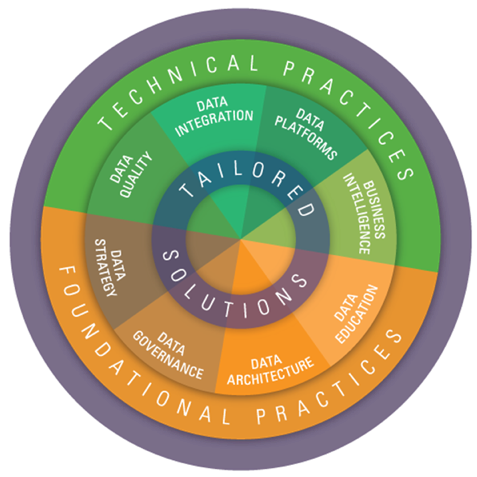
- Our unique approach leverages core competencies in both foundational and technical Data Management (DM) practices
IDMP+ refers to ISO IDMP, ICSR & SPL Standards